Wednesday, September 12, 2007
Colorado Public Company News:
Accelr8 (AMEX: AXK) ’06 REVENUE: ~ $213K APPROVED PRODUCT(S): None FOCUS: deliver bacterial identification and specific antibiotic susceptibility testing within 8 hours of specimen receipt. PIPELINE: development stage for the BACcel™-1.0 and the BACcelr8r™, based on its innovative surface coatings, assay processing, and detection technologies. Accelr8 also licenses certain of its proprietary technology for use in applications outside of its own products. NEWS: Announced accumulation of large data sets from prototype instruments and validating assays, next focus to ramp engineering to develop marketable product, Presented MRSA data at ASM meeting
Allos Therapeutics (NASDAQ: ALTH) '06 REVENUE: ~ ($32MM) APPROVED PRODUCT(S): None FOCUS: on the development of small molecule therapeutics for cancer PIPELINE: Pralatrexate (PDX) lead 2 Phase II for peripheral T-cell lymphoma and non-hodgkin’s lymphoma 3 Phase I for non-hodkin’s lymphoma (PDX plus gemcitabine), non-small cell lung cancer and T-cell lymphoma (PDX plu folic acid) NEWS: Presented at Bear Sterns conference yesterday, replay is (here), initiated enrollment for Phase I PDX plus folic acid study added to the Russel1 3000 broad market index.
Array BioPharma (NASDAQ: ARRY) – ’06 REVENUE: ~ $45MM APPROVED PRODUCT(S): None FOCUS: Cancer and inflammatory disease small molecule drugs. PIPELINE: 1 Phase II candidate ARRY886 MEK target, 6 Phase I candidates ErbB-2/EGFR, p38, KSP, MEK targets, 3 Pre-clinical candidates. NEWS: CEO Rob Conway presented at the TWP Healthcare conference last week. The webcast has not been posted yet, expect to find it (here), ARRY380 advances to Phase I trial
Aspen BioPharma (NASDAQ: APPY) ’06 REVENUE: ~ $1.2MM APPROVED PRODUCT(S): Manufactures and markets approximately 30 purified protein products primarily for use as controls by diagnostic test kit manufacturers and research facilities, to determine whether diagnostic test kits are functioning properly. FOCUS: Development of novel animal reproduction products for large worldwide markets. PIPELINE: Developing diagnostic products, such as AppyScore ELISA test and AppyScreen, for. It also provides three bovine reproduction products, SurBred, a blood test designed to determine pregnancy status in dairy cattle; StayBred, a luteinizing hormone analog for inducing ovulation in cows; and BoviPure FSH, a follicle stimulating hormone analog for ovulation and embryo transfer in dairy and beef cows. The company is developing two equine reproduction products, EquiPure LH, a luteinizing hormone analog for inducing ovulation in estrous mares; and EquiPure FSH, a follicle stimulating hormone analog for ovulation and embryo transfer in horses. NEWS: Presented at Roth Capital Partners conference, webcast available (here). Shares recently moved from trading on the OTC bulletin board under APNB.OB to the NASDAQ capital mrkts xchange under APPY.
Ceragenix Pharma (OTCBB: CGXP.OB) ’06 REVENUE: ~ ($4.7MM) APPROVED PRODUCT(S): EpiCeram® for treatment of atopic dermatitis (eczema) FOCUS: a biopharmaceutical company engaged in the discovery, development, and commercialization of a portfolio of small molecule cationic steroid antibiotic products in the fields of infectious disease and dermatology PIPELINE: preclinical candidates for neonatal barrier repair, cvc infections and burn dressing. Products are all based upon skin barrier repair and the ceragenin technologies NEWS: Recent recipient of the Frost & Sullivan's 2007 North American Technology Innovation Award for the field of Anti-Infective Coatings. Have not been able to consummate licensing deal for EpiCeram® by the forecasted dates previously released, commercialization is targeted for 2008. In vitro data indicate that CSA-13 may be effective to treat multi-drug resistant Pseudomonas aeuroginosa.
Encision (AMEX: ECI) ’06 REVENUE: ~ $11MM APPROVED PRODUCT(S): Sells a range of articulating instruments, such as scissors, graspers, and dissectors; fixed-tip electrodes; and suction-irrigation electrodes. In addition, Encision markets the Active Electrode Monitoring System monitor product line that is used in conjunction with the AEM Instruments. FOCUS: A medical device company offering a line of AEM Surgical Instruments, which prevents stray electrosurgical burns from insulation failure and capacitive coupling. PIPELINE: ? NEWS: Received warning letter from AMEX that ECI did not satisfy rule for continued listing. Reported a 3% decrease in quarter-over-quarter in net sales, ordering patterns of reorders for re-usable equipment was cited as a cause for the decrease. Will begin to manufacture its own disposable scissor inserts.
Heska (NASDAQ: HSKA) ’06 REVENUE: ~ $75MM APPROVED PRODUCT(S): Vet ABC-Diff Hematology System, ALLERCEPT® Allergy Testing and Treatment, CBC-Diff™ Veterinary System, E.R.D.-HealthScreen® Urine Tests, F.A. Granules, HemaTrue™ Veterinary System, i-STAT® Clinical Analyzers, Solo Step® Heartworm Tests, SPOTCHEM™ EZ Chemistry System, ThyroMed™ Chewable Tablets and Feline UltraNasal® Vaccines FOCUS: The company operates in two segments, Core Companion Animal Health, and Other Vaccines Pharmaceuticals and Products. The Core Companion Animal Health segment includes diagnostic and other instruments and supplies, as well as single use diagnostic and other tests, vaccines, and pharmaceuticals primarily for canine and feline use. PIPELINE: TBD NEWS: 3Q07 earnings call set for 7 November, call in info (here)
Pharmion (NASDAQ: PHRM) – ’06 REVENUE: ~ $239MM APPROVED PRODUCT(S): Vidaza® for myelodysplastic syndromes, Thalidomide® for multiple myeloma, erythema nodsum leprosum, Innohep® for deep vein thrombosis Refludan® heparin-induced thrombocytopenia FOCUS: Hematology and oncology PIPELINE: 4 Phase III candidates MDS, 1st and 2nd line MM, hormone refractory prostate caner, 5 Phase II candidates acute myelogenous, small cell lung cancer hematological malignancies, 4 Phase I candidates NEWS: Presentation from yesterday’s Bear Stearns conference will be posted (here), oral Azacitidine was granted fast-track status for myelosdysplastic syndromes, granted US Orphan Drug status for MGCD0103 for the treatment of hodgkin’s lymphoma
Replidyne (NASDAQ: RDYN) ’06 REVENUE: ~ $16MM APPROVED PRODUCT(S): None FOCUS: focused on discovering, developing, in-licensing, and commercializing anti-infective products. PIPELINE: 3 Phase III for bronchitis, sinusitis/pneumonia and acute otitis media, 2 Phase I for skin infections (impetigo and MRSA) and wound infections NEWS: Keep your eyes peeled for announcement of a new development partner for Faropenem.
Tapestry Pharma (NASDAQCM: TPPH) ’06 REVENUE: ~($17MM) APPROVED PRODUCT(S): None FOCUS: oncology, third-generation taxane prostate for pancreas and glioblastoma mutliforme PIPELINE: 1 Phase II candidate TPI287 for hormone refractory prostate cancer NEWS: Recently reported 2Q07 financial results, recently withdrew S-1 filing for proposed $40MM common stock offering based upon encouraging data presented at ASCO and is pursuing other financing opportunities.
Tuesday, August 14, 2007
ARRY-380 To Advance Into Human PI Trial

Array BioPharma, Inc. (NASDAQ: ARRY) plans to initiate a Phase I trial this fall for ARRY-380, a selective, orally-active ErbB-2 inhibitor for metastatic breast cancer patients and perhaps those who are resistant to Genentech’s (NYSE: DNA) HER2 targeting blockbuster Herceptin.
Wednesday, July 11, 2007
Action At Allos

Lot’s of action transpiring at Allos Therapeutics (NASDAQ: ALTH) since Paul Berns has taken over the reigns. We should expect a $50.5M financing to close tomorrow courtesy of Merrill, where 9M shares of common are being floated at $6 less underwriting fees and expenses, much of which to be gobbled up by Baker Brothers Life Sciences Capital. The lion share of the use-of-proceeds will be allocated to advancing pralatrexate (PDX) clinical work. Recently an independent data monitor completed an interim safety analysis and indicated PDX should continue in its pivotal Phase II trail in patients with relapsed or refractory peripheral T-cell lymphoma. A second interim safety analysis should be expected following 35 enrollees who have completed at least one cycle of PDX treatment. Finally, William Ringo was appointed to the Allos board. Mr. Ringo, most recently served as President and CEO of Abgenix and guided the $2.7B acquisition by Amgen.
Allos + NASDAQ = Great News!

Allos Therapeutics, Inc. (NASDAQ: ALTH) having met all minimum requirements was added to the NASDAQ Biotechnology Index. Being added to an index typically yields an uptick in share price and corresponding market cap as all funds, ETF’s, separate accounts etc. that mirror an index must purchase the newly added position, in this particular case the iShares NASDAQ Biotechnology Index Fund (AMEX: IBB).
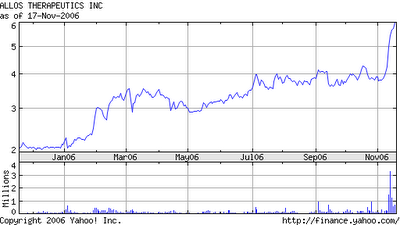
Taking a look at average daily volume of ALTH shares traded is ~275K however, today one may observe the buy spike of ~540K.
If you want to see a pretty one year chart take a look at this:
Pharmion: Bucks the Trend

After a fury of CO life science companies that have recently been acquired (e.g. Myogen/Gilead, RxKinetix/Endo and Sirna/Merck), Pharmion, Inc. (NASDAQ: PHRM) has reversed this local trend and gone out and purchased Cabrellis (privately held, San Diego, CA) for $55M with an additional $45M pending. Two additional payments of $12.5M are expected upon approval of amrubicin. Upon amrubicin's approval for a second indication in the US or EU an additional payment of $10 million will be made for each additional market.
Amrubicin (CALSED™) has been approved in Japan since 2002, for the treatment of SCLC and non-small cell lung cancer (NSCLC). Pharmion intends to initiate a Phase 3 registrational study in relapsed/refractory SCLC in the second half of 2007. Using data from these studies and supporting Japanese data, Pharmion expects to submit a NDA and a Marketing Authorization Application (MAA) in the EU for the treatment of relapsed/refractory SCLC during 2009.
The transaction adheres to the Pharmion model of late stage licensing and acquisitions; it may yield near term revenue (as early as 2010), complements the platform while expanding into solid tumor therapies and will also explore amrubicin in combination with their two epigenetic inhibitors.
Allos Therapeutics - Promising Data

Allos Therapeutics, Inc. (NASDAQ: ALTH) announced safety results from its Phase I/II trial of Pralatrexate (PDX) in patients with non-Hodgkin's lymphoma and Hodgkin's disease, and from its Phase II study of efaproxyn in patients with unresectable non-small cell lung cancer receiving sequential chemoradiotherapy (S-CRT).
Results of the PDX sudy indicate a higher incidence of stomatitis was observed in patients with elevations in pre-treatment homocysteine (Hcy) and methylmalonic acid (MMA). Little to no increase in stomatitis occurred in patients with Hcy and MMA less than 10 mM and 200 nM respectively. Patients with elevated Hcy and MMA who developed stomatitis with PDX did not develop advanced grade stomatitis after normalization of their Hcy and MMA with folic acid and vitamin B12 supplementation. Subsequent to this finding, all enrolled patients have received pre-treatment with vitamin B12 and folic acid, and normalization of Hcy and MMA levels has mitigated the incidence of stomatitis.
In July 2006, the FDA awarded orphan drug designation to PDX for the treatment of patients with T-cell lymphoma. PDX is a novel, small molecule chemotherapeutic agent that inhibits dihdrofolate reductase (DHRF), a folic acid dependent enzyme involved in the building of DNA and other processes. PDX was rationally designed for improved transport into tumor cells via the reduced folate carrier (RFC-1), and greater intracellular drug retention. These biochemical features, together with preclinical data in a variety of tumors, suggest that PDX has an enhanced potency and improved toxicity profile relative to methotrexate and other related DHFR inhibitors.
The efaproxyn study compared the safety and efficacy of efyaproxyn when administered with S-CRT in patients with unresectable NSCLC compared to data from a Phase III, Radiation Therapy Oncology Group (RTOG) study. Results of a 5-year survival analysis indicated that patients in the efaproxyn study exhibited superior survival to patients with similar characteristics in the RTOG study. Median survival of patients treated in the efaproxyn study was 20.6 months as compared to a median survival of 13.3 months for matched cases in the S-CRT arm of the RTOG study and 16.9 months in the concurrent chemoradiotherapy (C-CRT) arm of the RTOG study. The Kaplan-Meier estimates of 5-year survival rates for matched cases were 19% in the efaproxyn study and 10% in both the S-CRT and C-CRT arms of the RTOG study.
Efaproxyn is the first synthetic small molecule designed to sensitize hypoxic or oxygen-deprived areas of tumors during radiation therapy by facilitating the release of oxygen from hemoglobin, and increasing the level of oxygen in tumors. The presence of oxygen in tumors is an essential element for the effectiveness of radiation therapy. By increasing tumor oxygenation, Allos believes that efaproxyn has the potential to enhance the efficacy of standard radiation therapy.
Black Eye For Macugen

At their quarterly conference call OSI Pharmaceuticals, Inc. (NASDAQ: OSIP) announced a planned exit of their recent $650M Eyetech acquisition. OSI bought Eyetech primarily for the age related macular degeneration (AMD) drug Macugen. Macugen sales for the quarter rapidly and dramatically declined, reaching only $9M in sales relative to $87M for the previous two quarters. OSI seeks to maximize the value for Macugen and its research assets in the area via out-licensing, partnering or a sale of the business.
"We continue to believe that Macugen's product profile, our induction / maintenance strategy and promising data in the diabetic retinopathy area will ultimately result in a meaningful place for Macugen in treating retinal eye disease. However, it is evident that a key strategic goal behind our acquisition of Eyetech - that of generating positive cash-flow from the eye business in the 2006-2008 period - will not be realized."
Colin Goddard, Ph.D., Chief Executive Officer
The AMD market is crowded and is becoming ever more competitive. Visudyne, an AMD treatment from QLT, Inc. (NASDAQ: QLTI) and Novartis AG (NYSE: NVS), like OSI, has reported rapid sales erosion in 2006 of ~ $350M down from ~$380M. Genentech (NYSE: DNA) is on the AMD offensive with their off label sales of Avastin however, with planned royalties to Xoma (NASDAQ: XOMA), Lucentis appears to be accelerating as the choice for treatment. Lucentis is priced at $1,950 per dose and it is estimated that the average dosage will be five to seven treatments per year, yielding an annual cost of the treatment from $9,750 to $13,650. With as many as 15 million Americans affected by wet and dry forms of AMD Lucentis may easily achieve billion dollar sales and blockbuster status.
